CHEM 100/100G
SUMMER SCHOOL 2021
CHEMISTRY
Molecules that Changed the World
QUESTION ONE: Alcohol (answer ALL parts a-h):
(a) Is compound 1 an alcohol? Use the International Union of Pure and Applied Chemistry (IUPAC) definition of alcohol to justify your answer.
 (2 marks)
(2 marks)
(b) Draw the structure of ethanol. (1 mark)
(c) Is ethanol a primary, secondary or tertiary alcohol? (1 mark)
(d) The consumption of methanol is toxic to humans. The primary pathway to break down methanol inside our body involves two enzymes. Name the two enzymes, and briefly describe the roles of these two enzymes and the fate of the methanol. (5 marks)
(e) Ethanol is used as a biofuel. Discuss the factors that affect the net energy balance for the production and usage of ethanol-containing biofuels. (5 marks)
(f) Alcohol is used as a disinfectant (such as in hand sanitizers). Explain why disinfectants containing 100% alcohol are less effective than disinfectants containing 70% alcohol / 30% water mixture? (5 mark)
(g) Does carbonated alcoholic drinks speed up the absorption of ethanol? Explain your answer. (3 marks)
(h) A breathalyzer is an instrument that is used by law enforcement officers to check the amount of blood alcohol concentration during roadside breathing tests. Potassium dichromate and silver nitrate are essential components in a breathalyzer. Briefly describe their function. (3 marks)
Total = 25 marks
QUESTION TWO: Penicillin (answer ALL parts a-g):
(a) The structures of general penicillin and D-Ala-D-Ala are given below:
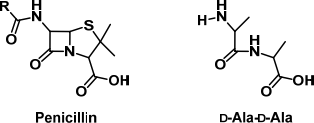
(i) Why did Alexander Fleming believe penicillin would not be of great clinical use? (1 mark)
(ii) Describe the mechanism of action of penicillin with reference to the structure of D- Ala-D-Ala. (6 marks)
(iii) One of the ways that bacteria try to become resistant to pencillins is by producing β- lactamase enzymes. What does β-lactamase do to penicillins? (2 marks)
(iv) Describe one way, other than the use of a β-lactamase inhibitor, to overcome bacterial resistance to penicillins. (1 mark)
(v) Copy the structure of penicillin into your answer booklet and circle TWO of the structural features essential for antibiotic activity. (2 marks)
(b) Describe the purpose of a Structure-Activity Relationship study. (2 marks)
(c) Describe one key difference between animal cells and bacterial cells. (1 mark)
(d) Describe the mechanism of action of the sulfa drug Prontosil. What is meant by the term ‘Prodrug’ . (3 marks)
(e) Define the term ‘semi-synthetic’ and explain the significance of the discovery of (+)-6- aminopenicillanic acid (6-APA) with regards to penicillin. (3 marks)
(f) What significant contribution was made by Edward Jenner in the fight against smallpox? (2 marks)
(g) State the names of two other classes of antibiotics besides the penicillins. (2 marks)
Total = 25 marks
QUESTION THREE: Gold (answer ALL parts a-d):
(a) Please answer the following multiple-choice questions focused on “Gold”. Write the letter of correct answer in your answer booklet alongside the question number.
i) Which one of the following is the densest element?
A: Copper
B: Gold
C: Lead
D: Iron
ii) The reduction of gold ions (Au+) to elemental gold (Au) in the last step of the gold recovery process involved which element?
A: Zinc
B: Copper
C: Nickel
D: Potassium
iii) Gold nanoparticles have applications in:
A: Health
B: Decorative
C: Biosensors
D: All of the above
iv) The inertness of gold makes it soluble only in:
A: Hydrochloric acid
B: Nitric acid
C: 3 : 1 mixture of Nitric acid : Hydrochloric acid
D: None of the above
v) Heterogenous catalysis:
A: Dissolves in solution
B: Dissolves in solution and the reaction occurs on the surface
C: Does not dissolve in solution and the reaction occurs on the surface D: None of the above
(1 mark each, 5 marks total)
(b) For each of the following statements, choose the correct answer TRUE or FALSE and write this in your answer booklet alongside the question number.
i) Gold is malleable enough to become translucent.
ii) Auranofin is used in the treatment of malaria.
iii) Silver and Copper are better conductors of electricity than Gold.
iv) An 18 carat gold alloy contains 50% gold.
v) Gold alloyed with >20 % copper produces white gold.
vi) Our current finical system is based on the “Gold Standard” in which the amount
of economic unit (money) is based on a fixed value of gold.
vii) Gold is a hard metal as indicated by a 10 on the Moh’s scale.
viii) Copper, Silver, Gold are all termed noble metals.
ix) Nanoparticles often display size-dependant properties i.e., colour change.
x) Gold is a poor conductor of heat.
(1 mark each, 10 marks total)
(c) Gold metal is dense, highly malleable and ductile, and has a bright yellow lustre. It is chemically inert and is unreactive towards air and water, furthermore, is a good electrical and thermal conductor and has low resistivity. Gold alloys are commonly used to attain more desirable properties. In TWO of the following applications, explain how at least three key chemical characteristics of gold underlie its use:
. as jewellery and for adornment
. as coins
. in electronics
. in dentistry
(6 marks)
(d) Potassium cyanide is an important chemical used in the processing of gold ore, according to the equation below:
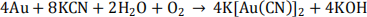
(i) What role does potassium cyanide play?
(ii) Why is the use of cyanide one of the controversial aspects of gold ore processing?
(iii) Identify and explain ONE way in which the Martha mine disposal area was designed to prevent cyanide leaching into the environment (4 marks)
Total = 25 marks
QUESTION FOUR: Nylon (answer ALL parts a-e):
(a) Name three common plastics that are low in cost and widely used. (3 marks)
(b) Plastics offer a wide range of properties and flexibility of designs not found in metals and ceramics. Name three basic characteristics of plastics materials. (3 marks)
(c) Plastic garbage is a widely recognized source of pollution. Over 1 billion tons of plastics waste is generated since 1950s.
(i) What are the three current methods of plastic waste disposal? (3 marks)
(ii) What are some advantages and disadvantages of each method? (6 marks)
(iii) Please comment on the current New Zealand plastics waste disposal approaches. (3 marks)
(d) The annual global production of polyethylene is around 80 million tonnes. The two most common forms of polyethylene are high-density polyethylene (HDPE) and low-density polyethylene (LDPE).
(i) Which of these do you think would be more appropriate for use in making milk bottles? (1 mark)
(ii) Explain the basis of your choice above. (1 mark)
(e) Nylon is a plastic, which is famously used in women's stockings since 1940.
(i) What are the names of the two chemicals (monomers) used to manufacture Nylon? (1 mark)
(ii) What is the significance of Nylon invention? (2 marks)
(iii) Why is Nylon a semicrystalline polymer? (2 marks)
Total = 25 marks