CM 2223 - Organic Chemistry II
Spring 2024
1. Draw the LINE STRUCTURE of an alkene with the formula C଼ H![]() that fits ALL of the following criteria: (8 pts)
that fits ALL of the following criteria: (8 pts)
口 disubstituted 口 4 sets of carbons
口 produces an achiral (but not meso) product when treated with RCOଷ H
2. Draw the LINE STRUCTURE of an isomer of your alkene from #1 that fits ALL of the following criteria: (8 pts)
口 disubstituted 口 3 sets of carbons
口 produces a meso product when treated with Brଶ
3. Draw the complete arrow-pushing mechanism for the reaction of your alkene from #1 with
RCOଷ H. Include all lone pairs and non-zero formal charges. Use dashes and wedges to indicate stereochemistry. Draw a box around the final product of the reaction. (10 pts)
Consider the following reactions:
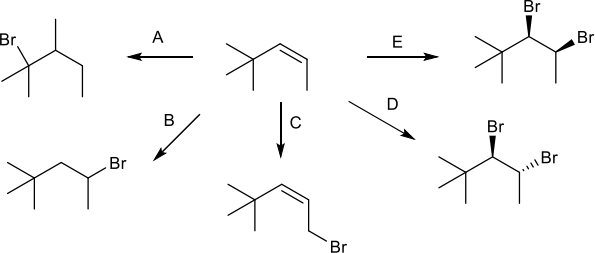
4. One of these reactions is not possible (at least as far as you know). Which one? Explain your answer. (5 pts)
5. Put an asterisk (*) by all of the stereocenters in the products shown above, and if the product is produced as a racemic mixture, write (+/-) next to the structure. (12 pts)
6. Fill in the reagents that are needed for each transformation. (10 pts)
|
A |
B |
C |
D |
E |
|
|
|
|
|
|
7. Reaction C could also produce 3-bromo-4,4-dimethylpent- 1-ene as a product. How does this happen? Explain your answer. (5 pts)
8. Draw the final product of the reaction of 1-methylcyclopentene with ozone, followed by dimethyl sulfide.
9. Which of the following alkenes could be used as the starting material to produce the compound in the box? Circle ALL that apply. (8 pts)
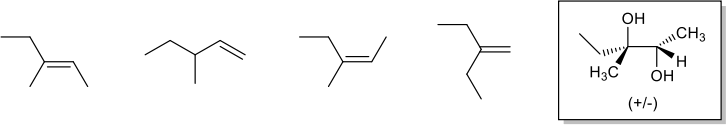
10. Choose one of the alkenes from #9. Which reagents would be needed for the reaction to produce the compound in the box? What kind of reaction is it (what is it called)? Is it regioselective? Is it stereoselective? Explain your answer. (10 pts)
11. One of the alkenes from #9 was treated with a peracid to produce an epoxide. The epoxide product has 6 peaks in the 13C NMR spectrum, and the 1H NMR spectrum has a doublet peak that integrates to 2H around 3.8 ppm. Which of the alkenes from #9 was used in this reaction? Explain your answer. (7 pts)
Consider the following reactions:
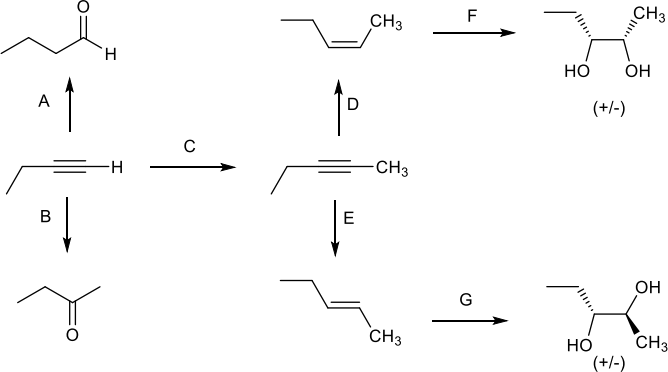
12. Fill in the reagents that are needed for each transformation: (14 pts)
|
A |
B |
C |
D |
E |
F |
G |
|
|
|
|
|
|
|
|
13. What is the relationship between the product of reaction D and the product of reaction E? Choose the ONE best answer. (5 pts)
Enantiomers Diastereomers Identical Regioisomers Resonance Forms
14. What is the relationship between the product of reaction F and the product of reaction G? Choose the ONE best answer. (5 pts)
Enantiomers Diastereomers Identical Regioisomers Resonance Forms
15. Explain the observed regioselectivity in reaction B. (10 pts)